
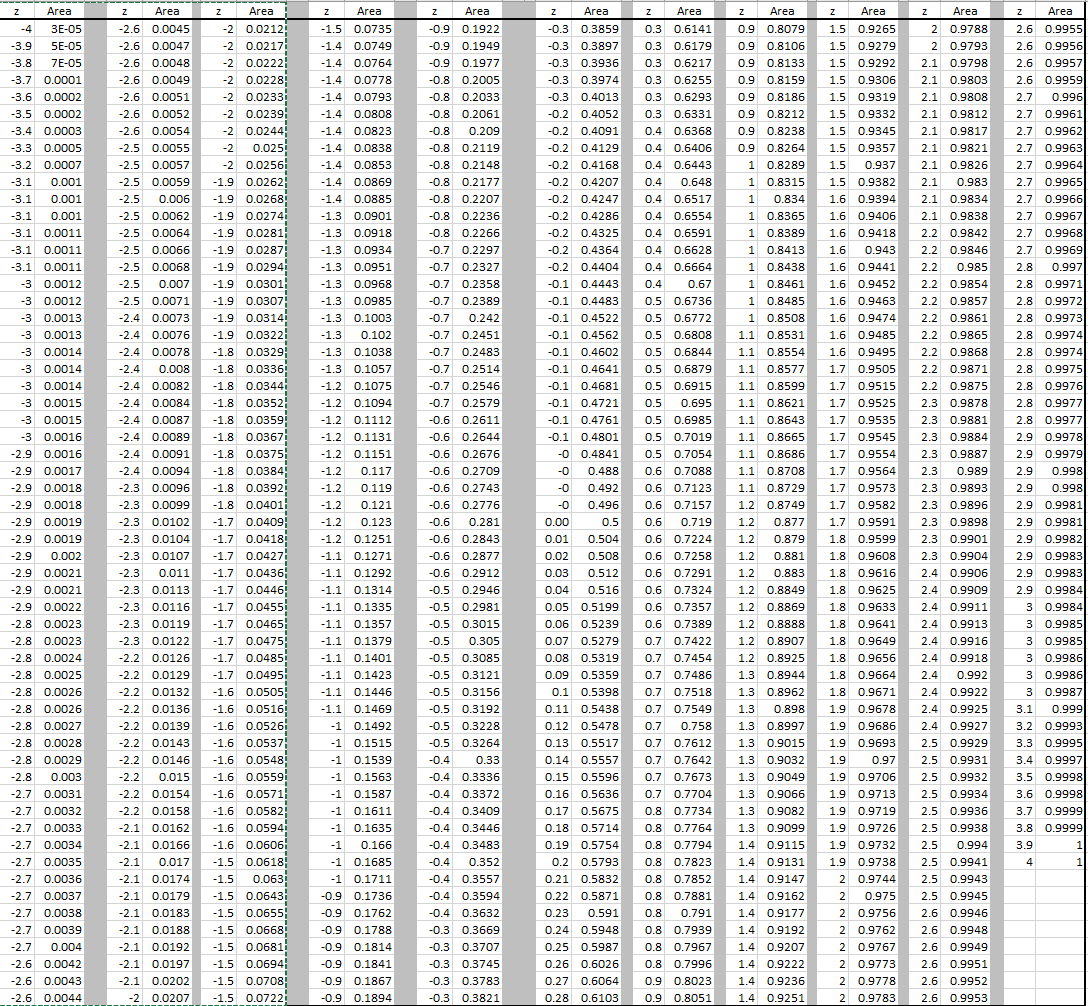
(in other words we reduced 100% to decimal form 1. We will let 6Li = x and 7 Li = 1-x we use 1 – x instead of 100 – x because the small number is easier to work with. Since I don’t know what the percentage are, I will have to use variables.ġ00% of Lithium is determined by these two naturally occurring isotopes. Determine the percent abundance of each isotope.Īw = + + Ħ.94 = + The atomic mass of lithium is 6.94, the naturally occurring isotopes are 6Li = 6.015121 amu, and 7Li = 7.016003 amu. Exact Masses and Molecular Formulae How to determine a molecular formula from an exact mass: Look up in a table, or Use a web-based calculator. Webster, Spectrometric Identification of Organic Compounds(Wiley, 1998), 6th ed.
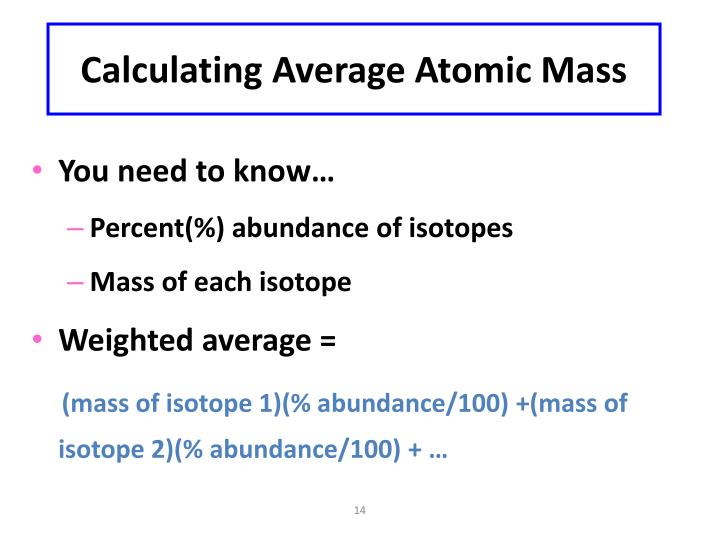
Atomic mass for Cu = 63.546Ħ3.546 = + Ħ5Cu = 1 – x = 1 – 0.6916 = 0.3084 x 100% = 30.84% How to determine a molecular formula from an exact mass: Look up in a table, or From R.

What are the percent abundances of the isotopes? Since the overall atomic weight for copper is not given in the problem, you must look it up in the periodic table to work this solution. If you look in the periodic table you will be able to check that our answer is correct!ģVerify that the atomic mass of magnesium is 24.31, given the followingĪtomic mass= + + ĭetermining the percent abundance of each isotope from atomic mass.Ĭopper exists as two isotopes: 63Cu (62.9298 amu) and 65Cu (64.9278 amu). 10.81amu so, the atomic weight of B = 10.81amu


 0 kommentar(er)
0 kommentar(er)
